By
Kevin E. Noonan —
 Earlier
Earlier
this month, Myriad Genetics filed patent infringement lawsuits against Ambry
Genetics (on July 9th) and Gene-by-Gene (on July 10th). Filed with the complaint in the Ambry lawsuit
was a Motion for Preliminary Injunction, and the brief supporting this motion
sheds light on the arguments Myriad will assert in its lawsuit (against Ambry
and, presumably Gene-by-Gene as well).
In
its introduction, Myriad reminds the Utah District Court what is at stake: that
the company had invested "over $500 million dollars" in developing
and commercializing its BRCA1 and BRCA2 genetic diagnostic tests. These tests have "revolutionized patient
care and provided medical diagnosis and treatment options never thought
possible," providing a test "of superior reliability and accuracy"
and that "has saved, and continues to save, countless lives." Myriad continues to have many claims in its
franchise (515 in 24 patents), including the ones specifically recited in its
complaint. Finally, Myriad takes the
position (one not completely supported by the Supreme Court's express language)
that the Court "found that, unlike isolated human genes, synthetic DNA is
man-made and is not a product of nature. Plaintiffs' remaining patent claims
covering BRCA1 and BRCA2 gene testing, including those at issue here, pertain
to synthetic DNA or methods-of-use, which were not affected by the Court's
decision, and remain valid and enforceable."
The
Statement of Facts provides access to the company's background on its website
and recites one important aspect of Myriad's business: as a result of its 16 years and one million
patients tested, Myriad has "an extensive database of genetic variant
information . . . [that] has allowed Myriad Genetics to further improve its
test quality by ensuring that over 97% of the patients tested with BRACAnalysis®,
who receive a report identifying a genetic variation, will be informed as
to the clinical significance of the variant." And the company emphasizes the pioneering
nature of its work:
Myriad Genetics has also invested heavily in creating from scratch the
market for breast/ovarian cancer genetic testing, including conducting
extensive clinical studies in support of medical industry guidelines regarding
hereditary cancer predisposition testing, developing a market of insurance
reimbursement, both public and private, for such testing, and promoting
physician and patient education surrounding the importance of hereditary cancer
awareness and testing.
 This
This
section of the brief also references Ambry's activities specifically relevant
to Myriad's patented technology (in particular, four tests identified as
BreastNext, BRCAPlus, CancerNext and OvaNext) and Ambry's announcement that it
would begin to provide BRCA testing. This announcement contained the information
that Ambry would offer its BRCA tests for $2,280 (compared with Myriad's
$4,040), a "significant" price drop, and asserted that "[w]hile
Ambry's tests do not offer the accuracy, quality and reliability of Myriad
Genetics' integrated BRACAnalysis® test, they present a significant
competitive threat as third-party payors, rather than patients and their
health-care providers, frequently decide where testing will be performed and
such payors are often not well-informed about the competitive quality of such
tests."
Turning to the nature of the testing, Myriad tells the District Court
that the difference in its claimed probes and PCR primers are that they
comprise "synthetically created complementary DNA molecules" that
differ from the genomic DNAs claimed in the claims invalidated by the Supreme
Court because "they
are not naturally occurring,[ r]ather, they are synthetic, laboratory-created
DNA carefully designed by man to achieve specific performance metrics." (While this is one interpretation of the Court's decision it is not the only one; indeed, the Court's basis for
distinguishing patent-eligible cDNA and patent-ineligible genomic DNA was not on
the basis of it being synthetic versus naturally occurring but that cDNA cannot be
found in nature, a subtle but real difference.) Nevertheless, Myriad contends
that "[c]reating synthetic DNA sharing sequence similarity with any
particular gene requires an application of detailed knowledge from the discovery
of that gene's structure," thereby placing its primers in that portion of
the opinion that indicates that "applications" of the knowledge of
the BRCA genes may be patent-eligible.
The
legal section of the brief sets forth the requirements for obtaining a
preliminary injunction: that there is a likelihood (not a certainty) that the
patentee would be successful on the merits of the patent infringement suit;
that the harm the patentee would suffer from infringement would be "irreparable"
and not adequately compensated by money damages; that the balance of the
hardships between the parties favors granting the injunction; and that the
injunction would be in the public interest. The brief proceeds to set forth arguments for each of these prongs
(albeit thinly for the hardship balance).
Myriad's
arguments for the likelihood it will succeed on the merits depend in large
part but not entirely on Section III of the Supreme Court's decision regarding
the availability of patent protection to Myriad based on applications of its
discovery of the human BRCA genes:
[T]his case does not involve patents on new applications of knowledge
about the BRCA1 and BRCA2 genes. Judge Bryson [of the Federal Circuit] aptly
noted that, "[a]s the first party with knowledge of the [BRCA1 and BRCA2]
sequences, Myriad was in an excellent position to claim applications of that
knowledge. Many of its unchallenged claims are limited to such
applications." Id. at 2120 (citing Ass'n for Molecular
Pathology, 689 F. 3d at 1349) (emphasis added).
Other
arguments include reference to its earlier (in the 1997 timeframe) "cease
and desist" activities against the University of Pennsylvania and other
academic labs, and that those earlier enforcement efforts "settled within
a year of filing." "Not one of those infringers raised a serious
contention as to the validity of Myriad's patents, and their quick exit from
the market is indicative of the validity of those patents," according to
this section of the brief.
Additionally,
Myriad takes advantage of the ACLU's decision to focus its efforts on the
isolated DNA claims, rather than the genetic diagnostic method claims, to
assert that this failure supports the statutory presumption that the claims the
company are now asserting are valid.
Turning
to claims for probes and primers, the brief takes away any sting or
disapprobation that could be associated by losing at the Court, stating that "there
was nothing untoward about Myriad having sought and obtained patent protection
over these newly discovered and isolated genes. Myriad's actions were
consistent with decades of patent practice and patent law, which the Supreme
Court refined with its decision." Further, the brief asserts that the Court's decision "reject[ed]
the argument that any synthetic DNA sharing any sequence similarity to natural
DNA is ineligible for patenting, despite the fact '[t]he nucleotide sequence of
cDNA is dictated by nature, not by the lab technician'":
That may be so, but the lab technician unquestionably creates something new
when cDNA is made. cDNA retains the naturally occurring exons of DNA, but it is
distinct from the DNA from which it was derived. As a result, cDNA is not a "product
of nature" and is patent eligible under §101, except insofar as very short
series of DNA may have no intervening introns to remove when creating cDNA. In
that situation, a short strand of cDNA may be indistinguishable
from natural DNA. Id. at 2119 (emphasis added).
Thus,
according to Myriad, all the claims it is now asserting "either require
the use of inventive DNA synthesized in a laboratory based upon knowledge about
the BRCA1 and BRCA2 genes (e.g., gene-specific probes, primers and
arrays) [] or pertain to such synthetic DNA compositions themselves, and
these compositions are patent-eligible under the Court's Myriad decision."
Finally,
addressing the patent eligibility of genetic diagnostic method claims the brief
bootstraps the (undisturbed) Federal Circuit decision that Claim 20 of Myriad's
'282 patent (relating to drug screening methods) are patentable, analogizing
the rationale for that decision (the non-naturally occurring nature of the
recombinant cells recited in that method) to Myriad's argument that the primers
and probes in its asserted claims are patent eligible under the Court's Myriad decision. (This argument does not
address evidence that the primer and probe claims are invalid under other
sections of the statute, inter alia, § 102
or § 103; see "Academic Amici Refute ACLU Falsehoods in Gene Patenting Debate".) The Court's Mayo decision is distinguished in
a footnote: "Myriad discovered a new biomarker, created new reagents and
techniques that could now analyze this new biomarker, and invented new methods
of determining a patient's risk of breast and ovarian cancer using these
reagents and techniques."
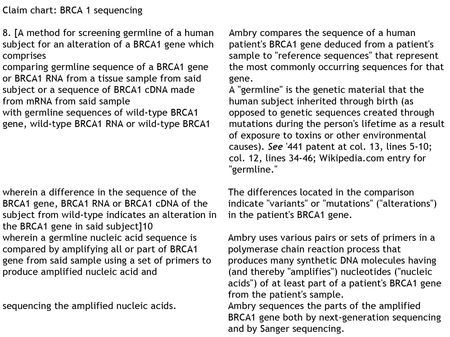
The
brief then sets forth Myriad's evidence that Ambry is infringing the asserted
claims. The specific acts Myriad asserts
are infringing are three: "(1) preparation of synthetic DNA samples for
BRCA1 and BRCA2 sequencing and analysis; (2) sequencing of BRCA1 and BRCA2; and
(3) large rearrangement analysis of BRCA1 and BRCA2." Myriad asserts that the process(es) used by
Ambry begin with DNA isolated from a patient sample that is fragmented and BRCA
gene sequences enriched (using "RainDance PCR Target Enrichment") and
then PCR amplified with BRCA gene-specific (exon-specific) primers to produce
synthetic DNA molecules that can then be sequenced. Conceding that the primers and probes used in
these processes "use natural DNA as inspiration," Myriad argues that "the
primer molecules themselves are entirely man-made; they are synthesized in a
laboratory" which is enough in Myriad's view to make these compositions
patent-eligible. Moreover, the amplified PCR fragments are themselves "100%
synthetic" insofar as they are produced in a laboratory and do not exist
in nature per se.
Myriad's brief then sets out claim charts for many of its
asserted claims (and makes arguments for all claims Myriad is asserting in the
litigation).

Myriad
also addresses the extent to which practice of "new" sequencing
methods fall within the scope of its claims from several sequencing generations
ago. The answer for Myriad is simple: the sequence of the BRCA gene exons "includes nucleotide position numbers
2201, 2731, 2430, 4427, 3232, 3667, and 4956" (the positions of mutations
indicative of a risk for breast or ovarian cancer), and thus are
infringing. And the brief reminds the District Court that Ambry also performs traditional Sanger sequencing (the state of the
art when the Myriad patents were filed).

Finally,
the brief addressed Ambry's large rearrangement analysis, which involves either
"(1) multiplex ligation-dependent probe amplification ('MLPA') analysis or
(2) chromosomal 'microarray' analysis." The MPLA assay requires the use
of "synthetic BRCA1 and BRCA2-specific probes" which are entitled to
the same patent-eligibility status that Myriad argues the District Court should confer
on synthetic DNA primers. The
chromosomal microarray analysis "necessarily requires hybridization of the
synthetic DNA created from a patient's sample DNA to a BRCA1- or BRCA
2-specific probe" which should infringe a patent-eligible claim according
to the same rationale.
On
balance, Myriad's arguments are based almost in their entirety on the
patent-eligibility and patentability of its claimed primers and probes and
methods of use thereof. These arguments
are certainly open to different interpretations and Myriad's success, in its preliminary
injunction motion and at trial will depend on how the Utah District Court (and
presumably the Federal Circuit) will interpret the Supreme Court's decision in
this regard. Myriad's assertion of this
interpretation of the Court's Myriad
decision also suggests that the company may once again be the impetus for the
Supreme Court to revisit the scope of its "product of nature" patent
ineligibility doctrine.
The brief's arguments regarding the
remaining prongs are much less specific for genetic diagnostic patenting. Regarding the irreparable injury prong,
Myriad recites "at a minimum" the following harms it anticipates
would arise from the Court denying its motion: "(1) price erosion and the
loss of the benefit of Myriad's established pricing strategy; (2) the loss of
market share; (3) reputational injury; and (4) loss of the benefit of the
remaining limited term of patent exclusivity and Myriad's business plans for
that period, as well as the inability to fully obtain its reliance interest
obtained by disclosing its discovery and investing hundreds of millions of
dollars to commercialize that discovery in exchange for a limited exclusive
right," citing Federal Circuit precedent on the relevance of these
factors. Robert Bosch LLC v. Pylon
Mfg. Corp., 659 F.3d 1142, 1152-54 (Fed. Cir. 2011) (however, this was a
decision in the context of a permanent injunction, where infringement had been
established). Ambry's purported cost for
its test ($2,280) is "deeply discounted" from Myriad's cost ($4,040;
a 46% discount). Myriad identifies the
fact that "third-party payors (such as insurers and/or HMO's) are
primarily responsible for deciding whether they will reimburse or pay for
testing, rather than the physician or the patient" to raise the risk of
price erosion, because "[t]hose payors will exert pressure on Myriad to
lower its prices in response to Ambry, and Myriad would be forced to do so in
some instances" (and this could get worse if other competitors entered the
marketplace). In addition, Myriad argues that it is not the only entity that would be
harmed: the brief asserts that Myriad has paid about $57 million to licensors,
which include universities and research hospitals, for whom losing this revenue
stream "will [negatively] impact their ability to fund ongoing
programs and new endeavors."
Myriad's arguments regarding market share have some
of the flavor of marketing itself: "[t]hrough its hard work and dedication, Myriad was
able to finalize this invention, secure licenses from the patent owners, and
develop a superior BRCA1 and BRCA2 test that not only created the market from
scratch, but exhibits superior methodology and unparalleled reliability." Ambry's activities amount to "free-riding"
on this effort, and Myriad will lose market share from Ambry's lower prices not
due to any advantages or benefits to patients but because "Ambry's
significantly discounted prices will result in some third-party payors
insisting that patients choose Ambry over Myriad solely because of cost and
regardless of the fact that Myriad offers a superior, far more reliable
product, and even if patients or physicians prefer to use Myriad." The evidence of the superiority of Myriad's
tests are based on the information Myriad has acquired from "over
1,000,000 patients tested" and the consequence that "Myriad []
provide[s] a clinically meaningful result for over 97% of the variants
identified, as opposed to approximately 70% using the publicly available
database." (The argument regarding
relative quality of the Myriad and Ambry tests are further developed in the
public interest section of the brief.) These
considerations are relevant to the irreparable harm prong of the test because "Myriad
has set its prices to reflect the higher quality of Myriad's test, including
the significant investments made in discovering the sequences of the BRCA1 and
BRCA2 genes, developing necessary technology to perform testing, building the
market and analyzing and characterizing variants in a proprietary database"
that results in these advantageously superior patient diagnostic outcomes.
Finally,
regarding reputational harm, the brief asserts that "Myriad's years of
experience and its built-in quality checks, including the fact that it has
developed proprietary DNA base calling software, have resulted in a near
perfect accuracy rate. Ambry's failure rate, in contrast, may be as high as 4% . . . Myriad has been able to further improve
its test quality by ensuring that its percentage of 'variants of unknown
significance' is less than 3%, compared to 25% to 30% in public databases." Ambry's entry into the marketplace creates a
risk to Myriad, because "[i]f Ambry is allowed to continue selling its
tests, which have a higher error rate than Myriad's and will result in many
more 'variants of unknown significance,' consumers will receive inconclusive or
even flatly incorrect results from those tests. However, because consumers
generally are not well-informed about the different test providers, in part
because third-party payors often select the provider based on cost, those
consumers are likely to associate those flawed results with Myriad." This outcome would be avoided if Ambry and
others are kept from the marketplace until Myriad's patents have expired,
because Myriad "has had no time or opportunity to distinguish its BRACAnalysis®
test and associated testing quality from competitors as it would if its
competitors were barred from entry until the patents' expiration." Myriad has relied on its patent exclusivity
to have the time to "finalize" its strategy for distinguishing its
tests from competitors but "is not prepared to implement those plans
immediately, which it would need to do in order to combat the effect of Ambry's
testing." And Myriad's is not the only reputation at risk, because
permitting Ambry's less accurate testing to be used on patients "would
also indirectly damage the reputation of the other patent owners, several of
which are respected research universities or hospitals."
The
"balance of the hardships" section of the brief is the shortest, amounting
to no more than an assertion that Ambry will suffer no harm, while the harm to
Myriad will be to "[a] significant part" of its business.
Finally,
the public interest section directly addressed the contrast between public
benefits that may be derived from lower prices and the public detriment that
Ambry's purportedly lower quality tests would produce. "While competition may serve the public
interest in the short term, the mere existence of a lower-priced, lower quality
option available from an infringer does not necessarily advance the broader
public interest," according to Myriad. In addition, "the public has a greater interest in acquiring new
technology through the protections provided by the Patent Act than it has in
buying 'cheaper knock-offs'."
The
argument that Ambry's testing poses a risk to the public is Myriad's first and
foremost argument. "[T]he public
interest at issue goes far beyond incentivizing invention. Precluding Ambry
from selling its less accurate test is critical, as allowing Ambry to proceed
results in significant public risk over the status quo where Myriad provides
testing of very high quality, accuracy and affordability." Moreover:
As discussed above, Myriad used its years in the market to perfect its
testing processes. This work resulted in a near-perfect accuracy rate. Ambry's
published accuracy rate of 96-99% means that as many as 4% (or 1
in 25) of patients tested with Ambry products will receive either a false
negative or a false positive. The false negative result, of course is of the
utmost concern. Assuming such an error rate, allowing Ambry into the market
will result in more patients believing incorrectly that they are not at
elevated risk, and not taking preventative measures that they otherwise would
take. Conversely, a patient receiving a false positive may well elect
preventative measures such as surgery when in fact there is no elevated risk. This untenable result can and should be avoided by issuance of an injunction.
According
to Myriad, the public interest requires patients to receive Myriad's tests "because
of Myriad's exclusive access to its proprietary and extensive database of known
genetic variants when making a comparison with a patient test sample,"
which permits Myriad to provide "definitive" results for more than
97% of patients, as compare with 70-75% of patients whose risk for breast or
ovarian cancer are assessed using Ambry's tests. And, ironically in view of the "second
opinion" genetic testing aspects of AMP
v. Myriad (and Congressional attention), Myriad argues that:
Thus, Ambry will inform 25-30% of patients tested that they have a
genetic variant, but will give them no further information about the clinical
implications of that variant. Because insurance will not reimburse for a
second, repetitive test, most patients will not be able to be tested
again. Thus, those patients and their
medical providers will be left to guess at an appropriate course of treatment. Some patients, knowing they have a genetic variant of unknown significance,
will assume the worst and undertake unnecessary prophylactic measures,
including potentially surgery, even though the underlying variant may be
benign. Allowing Ambry to proceed with its intent to enter the marketplace
would be injurious to the public interest, and Ambry should be enjoined from
doing so.
For better or worse, we live in a
world that Myriad made. In 1997, genetic diagnosis of cancer risk was in
its infancy; traditional genetic linkage analysis had been successfully
performed for diseases like Huntington's disease and other rare genetic
diseases. While some academic researchers had identified genes involved
in cancer, these were typically loss-of-function mutations in several (~5-6)
genes. BRCA gene analysis was different, because it predicted with ~90%
certainty that an affected woman would develop breast or ovarian cancer. These biological consequences suggested radical prophylactic methods for
prevention, each of which involved medical and personal costs.
Myriad was thus in the position of
having to convince doctors that their test was beneficial and was sufficiently
predictive to justify both the diagnosis and the treatment. It also
required that Myriad establish a network of genetic counselors capable of interpreting
the genetic information and counseling affected women (and in the context of
there being the "variations on unknown significance" that occurred at
much higher frequency then than it does 16 years later). And it required
Myriad to lobby governments and private payers that the cost of Myriad's test
was justified by the lower medical costs of prevention (which were not
inconsiderable) than treatment of breast or ovarian cancer (because the
personal costs were not the payers' problem and the alleviation of which not
their perceived responsibility).
Myriad asserts that it spent about
half a billion dollars to establish its business including all these ancillary
costs on top of the scientific and technology costs. Myriad did not spend
this money due to altruism; like it or not, basing a society on the principle
of "from each according to her abilities, to each according to her needs"
was tried, famously, in the Twentieth Century with disastrous results. But if we turn the clock back and let major medical centers in New York,
and Boston, and San Francisco, and New Haven, and Bethesda develop BRCA testing,
is there any hope or realistic expectation that women in Appalachia, or Oklahoma, or rural communities
throughput the country would have had better, or even equivalent access to such
testing?
Myriad says it has tested over one
million women. Is the fact that in our Imperfect healthcare system some
women have not been able to get tested enough to desire a world where either
that number or the demographic distribution thereof is significantly lower? That outcome is hardly an example of
promoting progress.


Leave a comment