By Mark Chael —
 Recently, Cytori Therapeutics, Inc. announced that the U.S. Patent and Trademark Office had issued a Notice of Allowance for claims directed to prosthetic devices seeded with adipose-derived stem cells. According to the press release, these claims are related to the use of Cytori's Celution® System for the treatment of bone diseases.
Recently, Cytori Therapeutics, Inc. announced that the U.S. Patent and Trademark Office had issued a Notice of Allowance for claims directed to prosthetic devices seeded with adipose-derived stem cells. According to the press release, these claims are related to the use of Cytori's Celution® System for the treatment of bone diseases.
Although not specified in Cytori's recent press release, a quick review of the online databases at the USPTO indicates that on June 23, 2009, a Notice of Allowance was issued in U.S. Patent App. No. 10/885,293 (U.S. Patent App. Pub. No. 2005/0058632) in response to the applicants' filing of amended claims, which included the following:
1. A method of processing adipose tissue that comprises a population of cells comprising adipose-derived stem cells for reintroduction into a patient, comprising:
removing unprocessed adipose tissue that comprises a cell population comprising adipose-derived stem cells from said patient;
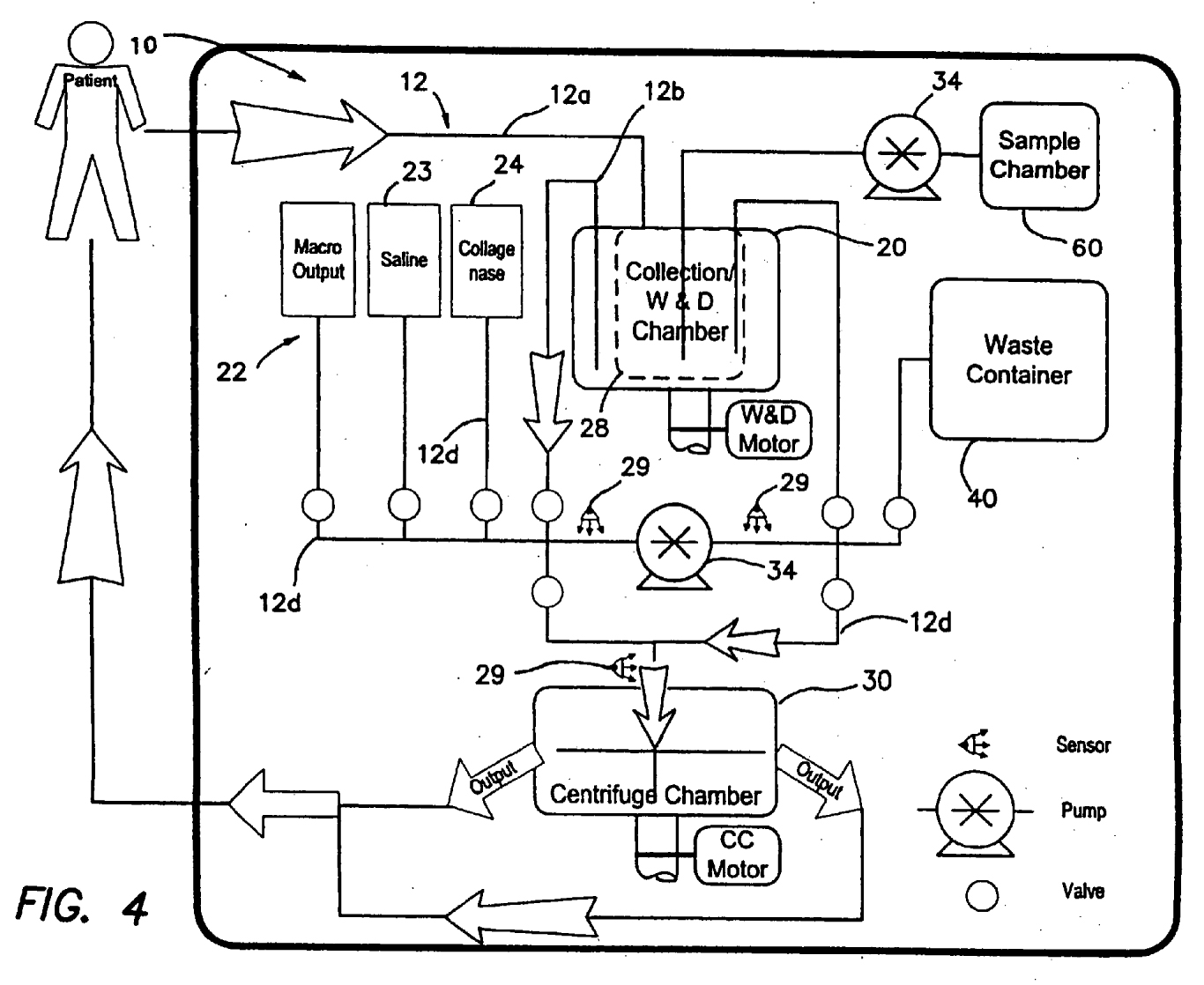
introducing the removed adipose tissue into a self-contained adipose-derived stem cell processing unit configured to maintain a closed system where said self contained adipose-derived stem cells processing unit comprises
a first filter that is disposed within said tissue collection chamber, wherein said first filter is configured to retain a first component of said unprocessed adipose tissue and pass a second component of said unprocessed adipose tissue, such that said first filter separates said first component from said second component, and wherein said first component comprises a cell population comprising adipose-derived stem cells and said second component comprises lipid, mature adipocytes, and saline;
a processing chamber, which is configured to receive said first component comprising said population of cells comprising adipose-derived stem cells from said tissue collection chamber, wherein said processing chamber is within said closed system;
a conduit configured to allow passage of said first component comprising said cell population comprising adipose-derived stem cells from said tissue collection chamber to said processing chamber while maintaining a closed system;
a cell concentrator disposed with said processing chamber, which is configured to facilitate the concentration of said first component comprising said cell population comprising adipose-derived stem cell so as to obtain a concentrated population of cells comprising adipose-derived stem cells, wherein said cell concentrator comprises a centrifuge or a filter; and
an outlet configured to allow the aseptic removal of said concentrated population of cells comprising adipose-derived stem cells;
introducing said concentrated population of cells that comprises adipose-derived stem cells into a prosthetic device, wherein said prosthetic device comprises a cell carrier portion and a cell carrier containment portion, and wherein said concentrated population of cells comprising adipose-derived stem cells is introduced into said cell carrier portion of said prosthetic device.
3. The method of claim 1, wherein said patient has a bone related disorder.
 Cytori and its proprietary system were profiled in 2006 by the San Diego Union Tribune. Also, as Patent Docs reported previously, Cytori is assembling a significant portfolio of patents and patent applications covering various uses of adipose-derived stem cells and methods for their isolation.
Cytori and its proprietary system were profiled in 2006 by the San Diego Union Tribune. Also, as Patent Docs reported previously, Cytori is assembling a significant portfolio of patents and patent applications covering various uses of adipose-derived stem cells and methods for their isolation.
According to information available from Cytori's website, the Celution® System consists of a cell processing device, single use consumables and related instrumentation and reagents. The cell processing device "rapidly and reliably delivers a clinical grade, mixed population of non-cultured adipose derived stem and regenerative cells at the patient's beside." Cytori does not sell these cells as a pharmaceutical product, unlike other stem cell commercialization enterprises, but rather commercializes the cell processing device, related instruments, consumables, and other reagents.

Leave a comment